First-in-human gene therapy
study for Canavan disease
treatment
Our Phase 1/2 gene therapy clinical trial offers
hope for children diagnosed with Canavan disease.
Trial Overview
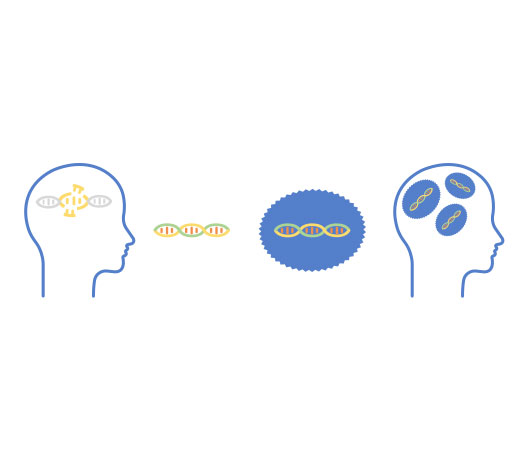
Myrtelle’s Phase 1/2 clinical trial is evaluating the safety and efficacy of gene therapy with this novel rAAV vector. In patients with CD, mutations in the ASPA gene impair normal myelination in the brain. Without myelin and its many functions, neurons are unable to properly transmit or receive signals in the brain. The gene therapy uses rAAV-Olig001 to deliver a normal copy of the ASPA gene, which encodes the enzyme aspartoacylase (ASPA). The gene therapy is intended to restore ASPA enzyme activity in the brain, restore myelin, and improve motor, cognitive and language development.
HOW IS THE GENE THERAPY ADMINISTERED?
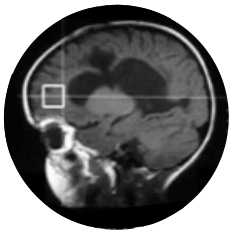
Myrtelle’s rAAV-Olig001 gene therapy is given as a single dose, one-time administration into two cavities, called lateral ventricles, of the brain, and hence, the route of administration is called intracerebroventricular. This method of gene therapy administration helps directly target the oligodendrocytes of the brain – the cells most involved in CD and where the functional copy of ASPA gene needs to be delivered.
PATIENT ELIGIBILITY
Patients must have a confirmed genetic diagnosis of Canavan disease, be between 3 months and 5 years old and meet all the eligibility criteria needed for clinical trial participation. Please visit clinicaltrial.gov for specific details.
WHAT WILL BE EXPECTED OF FAMILIES/CAREGIVERS
Patients and families/caregivers interested in this clinical trial should send an initial inquiry via [email protected]. After this initial contact, patients seeking to be enrolled will be invited to travel to the clinical site at Dayton Children’s Hospital in Dayton, OH for screening assessments approximately 1 – 3 months prior to gene therapy. If a patient qualifies for the study and is subsequently enrolled into the trial, they will return to the site approximately one week before the procedure for pre-surgical assessment. Following the gene therapy administration, patients will be monitored at the site for about 1 month and then followed for a total of 5 years post-treatment. The first year of this follow-up includes examinations and visits to Dayton Children’s Hospital at 1, 3, 6, and 12 months post-treatment, and then visits occur once every 12 months thereafter.
Each visit includes physical examinations, neurological testing, MRI and spectroscopy, laboratory tests, and other assessments as per the study protocol.
Learn More About Our Clinical Trial
STEPS TO ENROLLMENT
Parent/Doctor Contacts Myrtelle/Site
(Dayton Children’s Hospital)
Patient Identified
Site sends request for medical information
Principal Investigators (PIs) at the site review medical information
PIs identify appropriate potential candidates for enrollment
Patients requested to travel to site 1 – 3 months prior to gene therapy for consent and screening assessments
Patient is enrolled in study
Patient arrives at site approximately one week prior to gene therapy date for pre-surgical tests and evaluations
ARE THERE COST RELATED TO THE TRIAL?
All study-related expenses, including travel, lodging, and meals, will be covered for the protocol-specified treatment and visit schedule.
Research Timeline
1990s
Non-clinical studies investigate the ability to deliver the ASPA gene in-vivo.
1996
First ever human clinical study of ASPA gene delivery begins outside of the US, with liposome-based delivery developed by Dr. Paola Leone and colleaques.
1997
Human clinical study of liposome-based ASPA gene delivery extended to the USA.
2001
First ever human clinical study of ASPA gene replacement with AAV2 vector begins based on technologies developed by Dr. Paola Leone and colleagues.
2006
Canavan natural history data assembled by Dr. Paola Leone and colleagues.
2010
Novel AAV derived enabling cell-type specific targeting.
2016
Recombinant oligodendro-cyte-targeting AAVs (rAAV-Olig) characterized.
2018
Non-clinical data with rAAV-Olig developed to support human clinical study.
2020
USA IND opened for r-AAV-Olig001-ASPA gene therapy clinical study.
2021
Initial patient treated in first-ever clinical study of ASPA gene replacement specifically targeting oligodendrocytes.
STATEMENTS/DISCLAIMERS FROM MYRTELLE
- Myrtelle is committed to treating as many patients globally with Canavan disease as possible.
- We develop our clinical plans and strategies in alignment with global regulatory guidances, guidelines, and requirements.
- We are working to drive the development of our gene therapy in the quickest path to regulatory approval; the sooner the therapy is approved, the sooner it can reach and be available for patients.
- Drug availability and/or specifics of clinical trial design can impact how patients are enrolled in any clinical trial.
- We believe the best approach to the drug development is to conduct rigorous, well-planned clinical trials that evaluate robust endpoints, and as such, clinical trial protocols are properly designed then vetted by ethics committees/institutional review boards.
- The initial exploration of any therapy, including gene therapy, in a neurodegenerative disease setting is aimed at treating patients as early as possible before irreversible changes occur. This clinical development approach is reflected in the ongoing and future planned studies.
- We remain committed to expediting our clinical, regulatory, and manufacturing work in support of our mission to bring this important new treatment to patients with Canavan disease.
MYRTELLE OVERVIEW
Myrtelle Inc. is a gene therapy company focused on developing transformative treatments for neurodegenerative diseases. The company has a proprietary platform, intellectual property, and portfolio of programs and technologies supporting innovative gene therapy approaches for neurodegenerative diseases. Myrtelle has an exclusive worldwide licensing agreement with Pfizer Inc. for its Canavan disease program. For more information, please visit the Company’s website at: www.myrtellegtx.com.
Inquire About Our Clinical Trial
Please use the form below to submit inquiries regarding our clinical trial and research pipeline.